Harold C. Urey cooks up Deuterium for Thanksgiving!
And wins a Nobel Prize
During the busy and hectic holiday season, many of us sometimes have the unfortunate experience of being a bit late to important family events, including holiday affairs such as Thanksgiving dinner! But here is the story of an outstanding American scientist who had one of the better excuses in history.
Harold C. Urey
It would be ridiculous to attempt to quickly summarize the scientific achievements, or the admirable character of Harold C. Urey-for now, it will suffice that he was born in Indiana in 1893, and barely passed his entrance exams to attend high school (and he is not the only example like this among those who would, later in life, achieve world-class status in the sciences). While teaching in a Montana mining camp, he decided to attend college. From that point, Urey pursued a stellar scientific career, making fundamental contributions to human knowledge in numerous fields.
Oxygen isotopes
In 1931, a few years after Urey joined the faculty at Columbia University, the neutron had not yet been discovered. Rather, it was thought that isotopes were explained by additional protons (and electrons!) in the atomic nucleus. We now know that isotopes are atoms with the same number of protons, but different numbers of neutrons, in the atomic nucleus.
Additional isotopes of oxygen had been discovered in 1929, and this discovery had basically thrown the then-known table of elemental atomic weights slightly "out of whack." The accepted table of atomic weights was based on the relation between the elements of oxygen and hydrogen-and assumed the existence of only one isotope of oxygen (16O, which we now know has 8 protons and 8 neutrons in its atoms), and only one isotope of hydrogen (1H with a single proton comprising its nucleus). Because of the new isotopes of oxygen (17O and 18O, which have an additional neutron and an additional two neutrons in the nucleus, respectively), it was postulated that previously unknown isotopes of hydrogen might also exist... albeit in miniscule ratios to "ordinary" hydrogen. But no such isotopes of hydrogen had ever been detected.
When Urey read of this work in 1931, he quickly decided on a method of detecting such rare isotopes of hydrogen-his hypothetical, calculated, predicted variations in the atomic spectrum of "ordinary" hydrogen. A 21-foot spectrograph had just been installed at Columbia University, which could provide the resolution that would be needed.
Atomic spectroscopy
A quick word on atomic spectroscopy is in order. The general idea of a spectrum is of course well-known to anyone who has seen white light pass through a  prism. Atomic spectroscopy is a fundamental tool of chemical research, in which instead of a prism, one can substitute a pure sample of an element or molecule. Electrons in an atom exist in discrete energy states; when the outer electrons of an atom make a transition from a higher energy state to a lower state, they emit photons of a particular wavelength. This shows up in a spectrum as distinct "spectral lines." Likewise, electrons making a transition from a lower to a higher energy state absorb a photon, and a
prism. Atomic spectroscopy is a fundamental tool of chemical research, in which instead of a prism, one can substitute a pure sample of an element or molecule. Electrons in an atom exist in discrete energy states; when the outer electrons of an atom make a transition from a higher energy state to a lower state, they emit photons of a particular wavelength. This shows up in a spectrum as distinct "spectral lines." Likewise, electrons making a transition from a lower to a higher energy state absorb a photon, and a
spectrum will show absorption lines at specific wavelengths. When radiation is applied to a sample to excite the electrons, these "spectral lines" can be used to identify which elements and molecules are present in a sample-and even in light from distant galaxies. An isotope of hydrogen would show a shift in some of these spectral lines, different from "ordinary" hydrogen, in the visible part of the spectrum-and Urey had calculated where they should be, and was counting on this to identify new isotopes of hydrogen.
Liquid hydrogen in 1931
Almost everything was in order for Urey's work. Urey also knew that the concentration of the hypothetical isotopes in hydrogen samples would need to be much greater than normal. He required starting with 4 liters of liquid hydrogen-at minus 423.17 °F-in the year 1931. One of the only two locations in the United States capable of producing such was a new laboratory at the National Bureau of Standards, in Washington, DC.
F.G. Brickwedde, at this new laboratory-using Urey's calculations on how to distill the hypothetical new, heavier isotope of hydrogen into a concentrated, detectable fraction-evaporated 4000 milliliters of liquid hydrogen down to 1 ml and sent the sample back to Urey, who found nothing new in the spectrum. Another painstaking distillation, and another "null result"-but finally...
On Thanksgiving Day, 1931, Urey saw the expected increase in intensities of the "new" spectral lines, which had finally appeared in the concentrated sample-and deuterium had been discovered. We now know that while "ordinary" hydrogen consists of a proton and an electron, this new "deuterium" isotope of 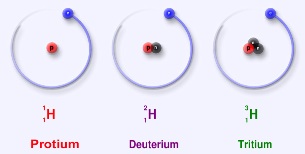 hydrogen was twice as massive, with a proton and a neutron in the nucleus. Urey returned to his home, late for Thanksgiving dinner, to inform his wife. Presumably, it was beneficial to have this good excuse. Also, the Nobel Prize awarded to him for this work, in 1934, hopefully helped make up for his tardiness!
hydrogen was twice as massive, with a proton and a neutron in the nucleus. Urey returned to his home, late for Thanksgiving dinner, to inform his wife. Presumably, it was beneficial to have this good excuse. Also, the Nobel Prize awarded to him for this work, in 1934, hopefully helped make up for his tardiness!
Deuterium
Deuterium has been fundamental in innumerable ways in answering questions about the Earth, the solar system, and the universe. Deuterium is not produced significantly in nature, and the fraction of deuterium observed in hydrogen (about 26 atoms of deuterium per one million total hydrogen atoms) seems constant wherever observed in the universe. This supports the idea of the Big Bang formation of the universe, in which all deuterium would have been formed at one place and time 13.7 billion years ago.
Meanwhile, Earth's own ratio of deuterium [156 atoms per million hydrogen atoms (ppm)] differs from that on Jupiter (the usual 26 ppm) but matches the ratio found in comets-supporting the idea that Earth's water has its origin from ancient comets. A quick example of deuterium's usefulness in planetary science: Hydrogen molecules with a deuterium atom are heavier and tend to  stay lower in a planetary atmosphere, where they are less likely to be broken up by solar ultraviolet radition and escape into space. So, the higher deuterium ratio measured in water in Venus's atmosphere indicates that the planet once had much more water than at present. Deuterium has provided innumerable clues in astronomical research, and in answering questions about Earth's past climate as well.
stay lower in a planetary atmosphere, where they are less likely to be broken up by solar ultraviolet radition and escape into space. So, the higher deuterium ratio measured in water in Venus's atmosphere indicates that the planet once had much more water than at present. Deuterium has provided innumerable clues in astronomical research, and in answering questions about Earth's past climate as well.
The Canadian CANDU nuclear fission reactor uses "heavy water" (D20) as a neutron moderator, while deuterium is (in)famously useful in fusion reactions, especially in combination with its heavier cousin tritium, the *other* isotope of hydrogen-and one day soon this will hopefully be a source of useful energy.
As deuterium reacts similarly to normal hydrogen in most chemical reactions, it is also very useful as a tracer to determine the pathways by which those reactions occur. The examples of benefits from knowledge using deuterium gained in this method are innumerable.
To close, one piece of advice: Do not attempt to explain your late arrival at family events by claiming the discovery of an unknown isotope of the most abundant element in the universe-this excuse already has been used.
Paul Bowersox feels like a heavier isotope of Paul Bowersox after Thanksgiving Dinner, and is a regular contributor to the ANS Nuclear Cafe.